The Rational Use of Medical Oxygen
Critical Considerations
In an emergency, give oxygen whenever it's clinically indicated; blood gas oxygen levels are not immediately necessary, and oxygen toxicity is not a primary concern In longer-term care, give oxygen only with known clinical goals and regular monitoring Use pulse oximetry for continuous monitoring; sudden changes in oxygenation may indicate misplacement of an endotracheal tube or an empty oxygen tank Administer oxygen in the smallest concentration and for the briefest period needed to achieve the desired therapeutic goal
In some patients, for example, those with dyspnea, hypotension, and sepsis, oxygen therapy is appropriate before the documentation of hypoxemia; In adults it's usually desirable to keep saturation values at 90% or higher while also avoiding inappropriately high saturation values; levels above 93-96 % may cause trouble in some CO2 retainers
Introduction
Probably no other drug is administered more frequently than oxygen. In fact, many physicians are no doubt so accustomed to giving oxygen that they forget that it is indeed a drug, complete with indications, contraindications, dosage ranges, and the potential for toxicity. What follows are guidelines for the sensible use of oxygen in the office and Emergency Department and for assessing the patient's response to oxygen therapy, with emphasis on the toxic complications that may occur when oxygen is used indiscriminately.
Historical Perspective
Although oxygen was discovered in the 1700s, its clinical use did not come into blossom until the early years of this century, when it was found to be helpful in patients with pneumonia. It became a not uncommon treatment in the preantibiotic era. In 1919, Haldane described the use of a loose-fitting mask for oxygen therapy, and in 1922, Stadie demonstrated the relationship between cyanosis and inadequate blood oxygenation.
The clinical benefits of oxygen therapy in patients with respiratory diseaseespecially pneumoniasoon became so well established that randomized prospective clinical trials were not considered to be ethical. So despite the fact that oxygen is probably the most commonly administered drug in hospitals, rigorous scientific data on its clinical benefits are limited.
Clinical Uses
Clinical situations in which oxygen is used generally fall into one of five major categories: medical and surgical emergencies, pulmonary disease, the perioperative period, the intensive care unit, and home care, called domiciliary oxygen use.
Medical and surgical emergencies. Virtually all patients with serious medical or surgical emergencies are given oxygen, usually by face mask, either when transported by ambulance or on arrival in the emergency department. They include patients with suspected myocardial infarction, severe asthma, congestive heart failure, and major trauma.
Although such patients may not be hypoxemic, giving them oxygen is generally harmless, at least until they can be assessed more fully, possibly with chest x-rays and arterial blood gas measurements. Never deny a patient oxygen in an emergency merely because of a theoretical concern about oxygen toxicity.
Pulmonary disease. Patients who are hospitalized with acute or chronic pulmonary disease may receive oxygen. They include patients with acute disease such as pneumonia, asthma, or pulmonary embolism as well as those with chronic obstructive pulmonary disease or pulmonary fibrosis, which develops over longer periods. Oxygen therapy may be needed for days or even weeks, so rational administration is especially important: oxygen must be given with clear clinical end points in mind and a means of evaluating therapeutic success.
The perioperative period. Supplemental oxygenoxygen in concentrations of more than 21%, the oxygen level in room airis almost always given during and after surgery, even to otherwise healthy patients. During operations carried out under general anesthesia, oxygen is usually given in concentrations of 30% or more, 30% being fixed as the lower limit on many anesthesia machines. For one to two hours after the operation, the patient will usually receive oxygen by face mask in the recovery room.
The rationale for the perioperative use of supplemental oxygen is that anesthetic agents, surgical muscle relaxants, artificial positive-pressure ventilation by machine, and the supine position all contribute to increased shunting in the lungs, which could result in hypoxemia without the use of supplemental oxygen. In addition, all general anesthesia requires continuous monitoring of the degree of arterial oxygenation by pulse oximetry, which allows early detection of anesthetic oxygenation problems resulting from mistakes such as esophageal or endobronchial intubation.
The intensive care unit. Most ICU patients are at some point intubated and ventilated mechanically by a positive-pressure volume ventilator. Oxygen concentrations are controlled with an air-oxygen blender that has a lower limit of 21% oxygen.
Although the spectrum of disease varies considerably from one ICU to the next, respiratory failure from causes such as pneumonia, adult respiratory distress syndrome, or sepsis is commonplace. Not infrequently, ICU patients are critically ill with multiple organ failure and require oxygen in high concentrationsmore than 70%as well as special techniques such as positive end-expiratory pressure to prevent hypoxemia.
ICU patients almost always have indwelling arterial catheters from which arterial blood samples for blood gas data can be obtained as needed, which permits a quick assessment of therapeutic response and thus the rational administration of oxygen. They typically need oxygen for prolonged periods, so its benefits must be weighed in the light of the risk of oxygen toxicity.
Home oxygen therapy. In recent years, many people have benefited from low-flow oxygen therapy at home, usually given by nasal prongs at flow rates of 1 to 3 L per minute, either at night or 24 hours a day. Portable oxygen supplies are readily available for use outdoors. An initial prescription is established by the patient's physician, and therapeutic effectiveness is monitored from time to time by pulse oximetry or arterial blood gas measurements.
The major drawback to home oxygen therapy is the expense, which can exceed $500 a month. Because of that cost, it's especially important that oxygen flow rates be kept to the lowest acceptable level by periodic monitoring.
Indications for Oxygen Administration
Acute and chronic disorders carry different indications for oxygen. In acute situations, supplemental oxygen is indicated when arterial blood gas studies reveal hypoxemia, defined as an oxygen tension less than 60 mm Hg. When pulse oxymetry is available, arterial saturation estimates may be used instead: an arterial PaO2 of less than 60 mm Hg corresponds to an arterial oxygen saturation of less than 90%.
In some acute situations, the clinical picture may suggest hypoxemia before objective evidence is obtained. Examples are dyspnea, hypotension, and sepsis. In such cases, oxygen therapy is appropriate before the documentation of hypoxemia. Indeed, in some cases the administration of low concentrations of supplemental oxygen for 24 hours or less without checking arterial blood gases is rational, as in patients with acute MI and those recovering from anesthesia and surgery. But with the general availability of pulse oximetry, noninvasive measurement of arterial blood saturation is now practical in all patients receiving oxygen.
The long-term administration of oxygen to patient with chronic disease requires special consideration of cost and efficacy. In general, several conditions should be met before oxygen is administered for more than 30 days: the diagnosis should be clear and the patient stable, the patient's illness should be judged to be refractory to other medical therapy by a physician knowledgeable in chest disease, and the patient's arterial PaO2 on room air, measured at rest and in the recumbent position, should be 55 mm Hg or less.
Once the need for oxygen therapy has been established, two issues must be addressed: By what route should the oxygen be given? And how much oxygen should be used?
Route
Oxygen may be given by one of five routes: face mask, nasal prongs, endotracheal tube, oxygen tent, and transtracheal catheter. The last two methods are used only occasionally and are mentioned only for completeness.
Oxygen delivery can by simple face mask, by nasal prongs, by face tent (for nasal surgery patients) as well as by a variety of other means, such as by endotracheal tube.
Image credits: http://www.lhsc.on.ca/resptherapy/rtequip/oxygen/index.htm
Face masks are probably the commonest means of oxygen delivery in acute situations. They are available with either fixed or variable oxygen settings and use the Venturi principle to entrain air into a stream of pure oxygen to produce the selected blend of room air and oxygen. At concentrations above 50 to 60%, special face masks with rebreathing bags are needed to avoid extremely high oxygen flow rates. If you need to know exactly what concentration of oxygen a patient is getting, not just the dial setting, you can use an airway gas monitor, for which a sampling catheter is fitted inside the face mask. A detailed discussion of face masks can be found in Wade's Comprehensive Respiratory Care: Physiology and Technique (St. Louis, C.V. Mosby, 1982).
Nasal prongs are a popular means of administering oxygen when relatively low concentrations are needed. They are typically used with oxygen flow rates of 1 to 4 L a minute. When the ventilator is set above 4 L a minute, the patient has the uncomfortable sensation of a blast of air going up his nose.
Nasal prongs have the advantage of being unobtrusive and allowing the patient to eat while receiving oxygen. The actual oxygen concentrations that a patient receives depends on so many factorsbreathing pattern, oxygen flow levels, and so forththat determination of the actual oxygen concentration in a given patient, when necessary, is best done by placing the sampling catheter of an airway gas monitor in the patient's nasopharynx.
At the other extreme, when oxygen concentrations in excess of 60 to 70% are needed, the patient is often in such severe respiratory failure that endotracheal intubation and mechanical ventilation are required. In such cases, the selection of oxygen concentration is only one factor among many in managing hypoxemia. Patients often benefit from a degree of positive end-expiratory pressure to keep their lungs expanded. Although too much PEEP can have detrimental effects, such as reducing cardiac output, the judicious use of positive end-expiratory pressure can sometimes allow the oxygen concentration to be adjusted downward, reducing the potential for oxygen toxicity.
Adjusting the FIO2
In many situations, after arterial blood gas results are obtained at a certain fraction of inspired oxygen (FIO2), you may wish to adjust the FIO2 to obtain the targeted arterial PO2. If the PO2 obtained on 30% oxygen is 65 mm Hg, for example, you may want to increase the oxygen concentration to achieve a target oxygen tension of, say, 90 mm Hg.
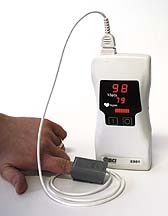
PULSE OXIMETRY
The rational administration of oxygen has been greatly facilitated by the advent of the pulse oximeter. A simple but fairly expensive instrument, costing $1000 to $10,000, the device consists of a probe and a microcomputer-based display system. The probe employs two light-emitting diodes (LEDs), one that produces infrared light and one that produces red light. Light from the two LEDs is picked up by a photodetector in the probe after the light passes through a vascular bed containing arterial blood. The ratio of the intensity of the red light and infrared light as detected at the peak of each pulse is calculated by the computer, which then displays a measurement of arterial blood saturation.
Pulse oximetry has two main advantages. First, it's noninvasive, requiring only a clip that attaches to a finger, toe, or earlobe. Second, since arterial saturation measurements are available beat by beat, you can promptly detect sudden changes in oxygenationas might occur, for example, with misplacement of an endotracheal tube or emptying of an oxygen tank.
Pulse oximetry has a few disadvantages other than the expense of the device. The probe may not yield reliable results when used on cold extremities, when perfusion is poor, or when the probe shifts in position, causing movement artifact. Second, pulse oximetry may be inaccurate in patients who have significant carboxyhemoglobinemiamost often found in heavy smokers. Finally, the results are expressed in terms of oxygen saturation, not the more familiar arterial oxygen tension.
Criteria for Initiating Oxygen Therapy
In acute situations
Any one of
With chronic disease
Chronic hypoxemia: PaO2 < 55 mm Hg
Principles of Management of Hypoxemia
1. Apply supplementary oxygen by face mask. Use 100% if patient is very sick.
2. Intubate and ventilate patient if clinically indicated.
3. Investigate the cause of hypoxemia and treat cause where possible (eg. hypercarbia).
4. Titrate oxygen therapy to achieve a particular target arterial oxygen saturation (e.g. 94%) measured using a pulse oximeter.
5. In intubated patients: Apply increases in Positive End Expiratory Pressure (PEEP) in conjunction with increases in inspired oxygen tension to counter hypoxemia due to shunting (e.g., ARDS, pneumonia). Avoid oxygen toxicity by avoiding hyperoxia.
OXYGEN TOXICITY
Clinical experience shows that although no patient should be denied appropriate therapy for fear of oxygen toxicity, oxygen should usually be administered in the lowest concentration and for the briefest period needed to achieve the desired therapeutic goal. Potential benefits must be considered in the context of potential toxicity, and whenever oxygen is given for longer than several hours, careful monitoring must be maintained.
For many years after it became widely used, oxygen was given freely and without an appreciation of its hazards. Several forms of oxygen toxicity have since become apparent, in particular retrolental fibroplasia, sometimes known as retinopathy of prematurity; carbon dioxide retention; absorption atelectasis; syndromes of pulmonary oxygen toxicity such as tracheobronchitis, acute respiratory distress syndrome, and bronchopulmonary dysplasia; and oxygen toxicity due to hyperbaric oxygen administration.
Retrolental fibroplasia. Retrolental fibroplasia (retinopathy of prematurity) occurs most commonly in premature infants, generally under 2 kg in weight, who have received high concentrations of oxygen after delivery. Such aggressive oxygen therapy can cause neovascularization of the retina, followed by the development of fibrous tissue behind the lens. The disorder is usually bilateral, and complete blindness can result.
Management of infants at risk is facilitated by monitoring umbilical blood gas oxygen tensions, pulse oxymetry, or transcutaneous oxygen tensions. Criteria for identifying newborns at particular risk continue to evolve; one set of criteria include infants receiving more than 40% oxygen, those receiving oxygen for more than seven days, and those who are especially premature, with more-immature retinal vasculature. Unfortunately, retrolental fibroplasia remains a problem in many premature infants despite vigorousness efforts at prevention. Periodic retinal examinations, beginning at about six weeks of age, are generally recommended, but policies for both monitoring and treatment remain controversial. For an up-to-date discussion of these issues, see the recent editorial in The Lancet (North Amerchol edition, vol. 339, p.691, 1992).
CO2 retention. Some patients who have chronic obstructive pulmonary disease depend primarily on hypoxia for their drive to breathe. when such patientsknown as CO2 retainersare given high levels of oxygen, their drive to breathe is reduced and arterial carbon dioxide tension may increase significantly. Be especially cautious about giving generous amounts of oxygen to COPD patients who have PaCO2 values already elevated to 45 or 50 mm Hg or higher when breathing room air.
Patients susceptible to CO2 retention are best given oxygen in carefully managed steps: for example, you can begin with 24% oxygen and gradually increase the concentration by 4 to 6% each time arterial blood gas analysis reveals continuing hypoxemia without increased hypercarbia. Pulse oximetry can be especially useful in such patients, by reducing the number of necessary needlesticks; patients are unlikely to lose their hypoxemic respiratory drive as long as SaO2 remains less than 90%. In some patients with severe COPD, hypercapnia may be so severe that intubation and mechanical ventilation are required.
Absorption atelectasis. When patients are given high levels of oxygen for long periods, the relative absence of inert "splinting" gases such as nitrogen or, in the operating room, nitrous oxide may lead to so-called absorption atelectasis. The disorder can result in impaired gas exchange, which manifests as hypoxemia when oxygen levels are reduced. A simplified explanation of absorption atelectasis is that the absorption of alveolar oxygen is so complete that without splinting by inert gases, the alveoli collapse.
Cytotoxic pulmonary oxygen toxicity. High concentrations of oxygen can be toxic to all cells, and those in the respiratory tract are especially vulnerable, because they are exposed to the highest concentrations of oxygen. Current thinking holds that high oxygen concentrations produce an accelerated rate of partially reduced oxygen productssuperoxide ions, hydrogen peroxide, and hydroxyl radicalswhich are normally generated as by-products of cell metabolism. These so-called free radicals react with cell components, producing damage that varies with the type of cell affected.
Cytotoxic pulmonary oxygen toxicity may be deadly to pulmonary capillary endothelium. Alveolar type I epithelial cells are also sensitive to oxygen toxicity. The resulting lung injury manifests on the cellular level as interstitial edema, proliferation of fibroplasts and type II epithelial cells, the presence of inflammatory cells, and other changes. Clinically, cytotoxic pulmonary oxygen toxicity can be divided into acute syndromes, such as tracheobronchitis and ARDS, and chronic syndromes, such as bronchopulmonary dysplasia.
Tracheobronchitis is not itself life threatening, but it can lead to complications such as pneumonia as a result of decreased clearance of foreign matter. ARDS, which may be caused by many other factors in addition to oxygen toxicity, is characterized by alveolar and interstitial edema; management may require intubation and positive-pressure ventilation with PEEP.
Cytotoxic pulmonary oxygen toxicity appears at a threshold of about 0.6 atm, or 60% inspired oxygen concentration at sea level, although symptoms of toxicity have been found in healthy volunteers at oxygen concentrations as low as 35%. Clinically, the risk of cytotoxicity should be considered whenever oxygen concentrations of 50 to 60% or greater are used for extended periods.
Hyperbaric oxygenation. Hyperbaric oxygen therapy is given at specialized centers, principally for the management of carbon monoxide poisoning and the treatment of certain infections. Besides the usual concerns that surround hyperbaric medicinefire hazards, decompression sickness, and nitrogen narcosis, for exampleexposure to oxygen at pressures above 3 atm (2300 mm Hg) for periods as brief as 30 minutes can result in seizures. Longer exposure can produce direct toxicity in cerebral tissue with permanent sequelae. Hyperbaric medicine specialists are well aware of those issues, however, and they follow careful protocols to minimize complications.
A BASIC GUIDELINE
No patient should be denied appropriate oxygen therapy for fear of oxygen toxicity, particularly in emergency situations when hypoxemia may be present. Although specific needs vary from patient to patient, in adults it's usually desirable to keep saturation values at 90% or higher while also avoiding inappropriately high saturation valueslevels above 93-96% may be potentially dangerous in some CO2 retainers, for example.
SUGGESTED READING
Alexander CM, et al: Principles of pulse oximetry: Theoretical and practical considerations. Anesth Analg 68:368, 1989.
Deply DT: Developments in oxygen monitoring. J Biomed Eng 10:533, 1988.
Doyle DJ: Rules-of-thumb for prediction PO2 changes. Intensive Care Med 15:253, 1988.
Fisher AB: Oxygen therapy: Side effects and toxicity. Am Rev Respir Dis 122:61, 1980.
Fulmer JD, Snider GL: National conference on oxygen therapy, American College of Chest Physicians/National Heart, Lung, and Blood Institute. Chest 86:234, 1984.